Ocean Acidification
In ten million years, the acid level in the ocean was balanced. Within that environment, thousands of diverse creatures existed and prospered. However, within the last couple of centuries, that balance has been slowly broken: the ocean has gone “sour”, or more accurately, has significantly been acidified.
1. What is seawater acidity?
Most liquids are either acidic or basic. This nature is demonstrated on a scale called pH degree. For example, lemon juice is an acidic liquid, and toothpaste is a basic one.
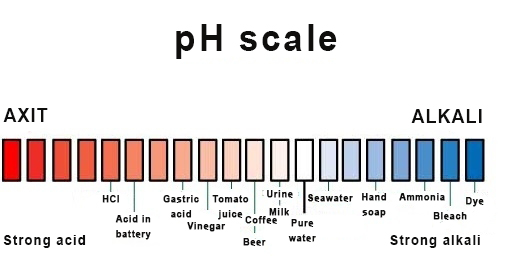
Usually, seawater is slightly basic, which means it is less acidic than freshwater. Unfortunately, when the ocean absorbs a large amount of carbon dioxide (CO2), the baseness of seawater increases and its acidity drops, negatively affecting the natural environment of many marine creatures.[1]
2. Why is the ocean acidified?
First of all, we need to understand that the ocean is a giant carbon tank. Carbon dioxide (CO2) in the atmosphere mixes into the ocean through the surface, and then moves to deeper layers through physical and biological processes. Here, some creatures use CO2, such as algae and seagrass, to photosynthesize. Due to that, the ocean has the ability to absorb a great amount of CO2 from the atmosphere. In the period from 2002 to 2011 alone, oceans have absorbed above 25% of CO2 emission that came from burning fossil fuels, land use and cement production over the world.[2]
Initially, scientists considered this a positive aspect; since when the ocean absorbs CO2, its level in the atmosphere will decrease and thus global warming will be slowed. However, in the last decade, they have realized that the excessive amount of CO2 absorbed by the ocean has changed its chemistry. Let’s take a look at how CO2 mixes into seawater.
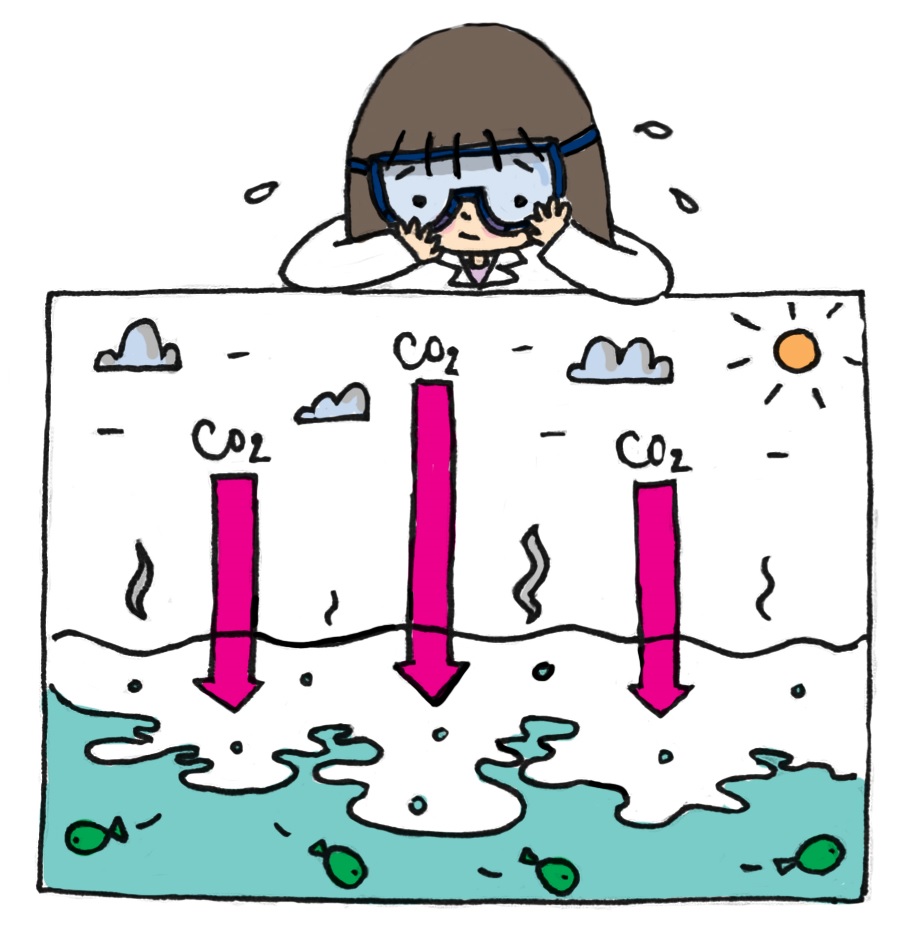
Let's look at the process of carbon dioxide dissolving into sea water.
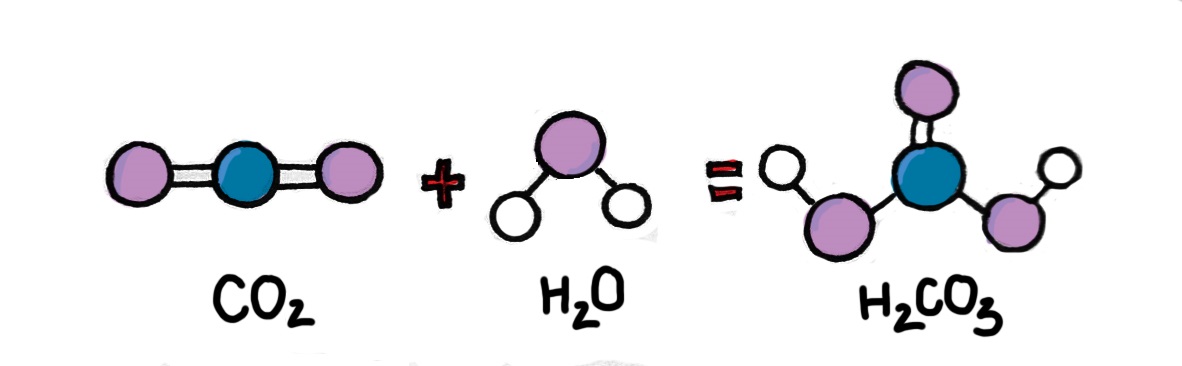
Firstly, CO2 reacts with water and creates carbonic acid (H2CO3-). Carbonic acid continues to release hydrogen ions H+ and bicarbonate molecules (HCO3-). The bicarbonate molecules continue to break up into hydrogen ions H+ and carbonate ions (CO3-). The increase of H+ decreases the pH of seawater, which means the seawater’s acidity rises.
3. What is happening?
The main component of the skeletons and shells of some marine creatures like corals, oysters, clams, sea urchins, starfish, is calcium carbonate (CaCO3). In order to create calcium carbonate, they use calcium ions (Ca2+) and carbonate (Ca2+) from the seawater. However, the acidification of the ocean, or the increase of H+, is halting this process.
How so? In reality, some H+ ions can combine with carbonate (CO32-) in the seawater to form bicarbonate molecules (HCO3-). Carbonate therefore cannot combine with calcium ions to create calcium carbonate (CaCO3), thus causing many creatures to being unable to develop their shells and skeletons. Their shells and skeletons become weak, prone to being brittle and breaking.

The most affected creatures are:
- Molluscs with shells (example: clams, oysters)
- Creatures with exoskeletons (example: starfish)
- Ecosystems reliant on skeletal structures (example: coral reefs)
Therefore, it’s evident that the absorption of CO2 has changed some processes underwater: acidification has appeared and affected the process of forming shells and skeletons of marine creatures. In reality, the amount of CO2 in the atmosphere has significantly increased since the beginning of industrialization. This is the result of using too much fossil fuel for industrial purposes, from transporting, producing, to consuming, along with other problems such as deforestation. Researchers have also shown that the pH degree of seawater has decreased from 8.2 to 8.1 in this period.

Currently, this process is still happening and possibly going to worsen if humans all over the world do not act promptly to cut down on CO2 emission.
In Vietnam
According to global reports about CO2 emissions’ ocean impact scenario, the East Sea will be gravely affected. Many regions in the East Sea will have a lower pH level of 0.3, which means a high level of acidification. Currently, only 80% of our corals still remains, by 2050, the number will decline to 50%, and by 2100, only 25%. In short, without preventive measures, sea creatures in general will be negatively affected, and corals in particular will be gravely destroyed by climate change. [3]
4. Let's act
The best immediate action you can take is decreasing your daily CO2 emission. Save energy by turning off lights when they are not in use, walk or bicycle instead of driving a car, support clean energy programs (solar and wind energy)

Start discussing ocean acidification with others. Many are still unaware of the issue, or assume it’s the scientists’ responsibility! Therefore, try to help them understand the impact of ocean acidification and what we can do to protect the beautiful ocean!